Contaminants
NORM Decontamination
NORM scaling is a concentrate of naturally occurring radioactive material that comes from sedimentary rock and precipitates out and accumulates during production and contains higher than background levels of radiation.
Why is NORM a problem?
NORM Scale Decontamination
Current Industry Practices
Why is NORM (Naturally Occurring Radioactive Material) a problem?
The Earth’s crust is radioactive. As a result, many naturally occurring materials contain radioactive elements (radionuclides).
While the level of individual exposure is usually negligible, some circumstances arise wherein the concentration of primordial radionuclides reach a level that demands regulation and control.
Radioactive materials which occur naturally and expose people to radiation are known as “NORM” (naturally occurring radioactive material). Exposure to NORM is often increased by human activities (e.g., burning coal, making fertilizers, and oil and gas production operations). One of the main industries with an aqueous TENORM (technologically enhanced naturally occurring radioactive material) problem is the petroleum industry.
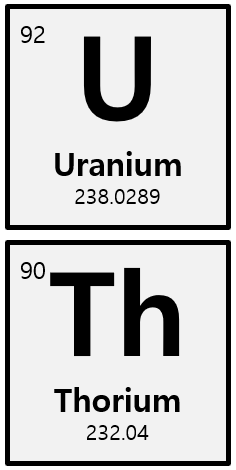
The radionuclides identified in oil and gas streams belong to the decay chains of Uranium 238 and Thorium 232. These elements are not mobilized from the reservoir rock that contains the oil, gas, and formation water. When the produced water is brought to the surface it contains Radium, together with an abundance of other cations, mainly alkaline earth compounds. The highly toxic nature of Radium renders the effective separation of these radiotoxic substances from non-toxic compounds highly desirable.

In general, the extraction constants of Ra2+ are lower than those of the other alkaline earth cations. This indicates that amino carboxylic acids (and their respective salts such as EDTA) cannot function as selective2+ extractants in waste water streams containing competing alkaline earth cations.
FQE® NORM-Clear™ and FQE NORM-Precip have been designed to address these highly challenging difficulties and to focus on efficient Radium removal through selective extraction and preferential adsorptive fractional precipitation.
These techniques effectively target Radium for expedient and cost effective control of NORM scales and contaminated waters.

White Paper
NORM Decontamination
We recently completed the world’s first specialized NORM chemical application on a Depropanizer in Texas, achieving exceptional results.
NORM Scale Decontamination with FQE NORM-Clear
FQE NORM-Clear has been developed with selective extractants designed for complexation of Ra2+. FQE NORM-Clear will preferentially form complexes with Ra2+ compared to the alkaline earth cations Na+, K+, Mg2+, Ca2+, Sr2+, and Ba2+, of which a significant excess exists. Of all the alkaline earth elements, Ra2+ is the most challenging.
FQE NORM-Clear is a water-soluble product, and can be applied as an additive at the termination of standard equipment decontamination procedures. It can be dosed into steam vapor, allowing the condensate to coat the affected equipment surfaces, or applied through an aqueous circulation. Concentrations of 0.5–1.0% in vapor phase condensation or 1.0–2.0% in aqueous circulation is recommended. Higher levels of radiotoxic scaling will require additional product to address the descaling process. In circulation processes, application temperatures of 120–150°F (49–66°C) are recommended.

Remove Dissolved Radioactive Salts with FQE NORM-Precip
FQE NORM-Precip functions by co-precipitation and enhanced coagulation of radiotoxic Radium and other radioactive salts as separated water-insoluble salts. The water-insoluble salts can then be removed by gravity differential or other means of filtration. The radioactive-free water produced from FQE NORM-Precip application can be recycled back to process or disposed of through normal wastewater treatment facilities, thereby drastically reducing waste water costs.
As a water soluble product, FQE NORM-Precip is applied directly to water streams containing dissolved radioactive materials. It is recommended that FQE NORM-Precip be added to the affected water prior to the dewatering process.
Collection of the remaining radioactive solids removed from the water must be properly handled according to local statutory regulations.
Current Industry Practices
One of the standard industry accepted practices for NORM removal is high pressure and ultra-high pressure water jetting up to 40,000PSI for the physical/mechanical removal of the NORM scale. This process is both time and personnel intensive, often requiring careful control and containment of the scale solids. In addition, mechanical methods are not often capable of reaching areas within equipment that can be accessed through a liquid chemical application and treatment.
Reverse osmosis is the process of using pressure to force a water component through a semi-permeable membrane while retaining ions of dissolved solids. This practice is useful in desalination of contaminated waters with no utility for the extraction of the radioactive salts from contaminated hard surfaces such as soils or equipment. In waste water purification, the methodology will not target radioactive substances, separating all ions of the solute present in the water. The membranes can blind-off and are often expensive in practice.
Chelation processes suffer from the same inefficiency of non-specificity as reverse osmosis. The success of such processes is dependent on the tendency of the metal ion to form a ligand bond with the chelating chemical in use. Since Radium has the lowest affinity for forming a complex, all other metal cations will complex before Radium, requiring more chelating agent to achieve the reaction. The requirement for such an excess of chelating agent for Radium chelation is problematic when large amounts of other metal compounds are also present.



